English Summary
The Netherlands Commission on Genetic Modification (COGEM) is the government’s scientific advisory body on matters concerning genetic modification. COGEM has two statutory tasks: 1) to advise the Minister of Infrastructure and Water Management and the ministry’s regulatory bodies and licensing authorities on the possible risks to human health and the environment of work with genetically modified organisms (GMOs), and 2) to inform the government on the ethical and societal aspects of genetic modification.
COGEM’s remit covers all areas of biotechnology, from agriculture to medical applications and from laboratories to the large-scale and commercial introduction of GMOs. COGEM does not give advice on issues relating to food or animal feed safety or on the possible risks to test subjects and patients in clinical trials or the authorisation of medical products. The Commission consists of no more than 40 members and associated members divided across three subcommittees and is supported by a secretariat of eight people.
Advisory and policy reports
In 2024 COGEM published a number of advisory reports and policy reports. The COGEM subcommittees on Agriculture (ScL) and Medical and Veterinary Aspects (ScMV) both prepared 37 advisory reports. Four publications were prepared jointly by two or three subcommittees.
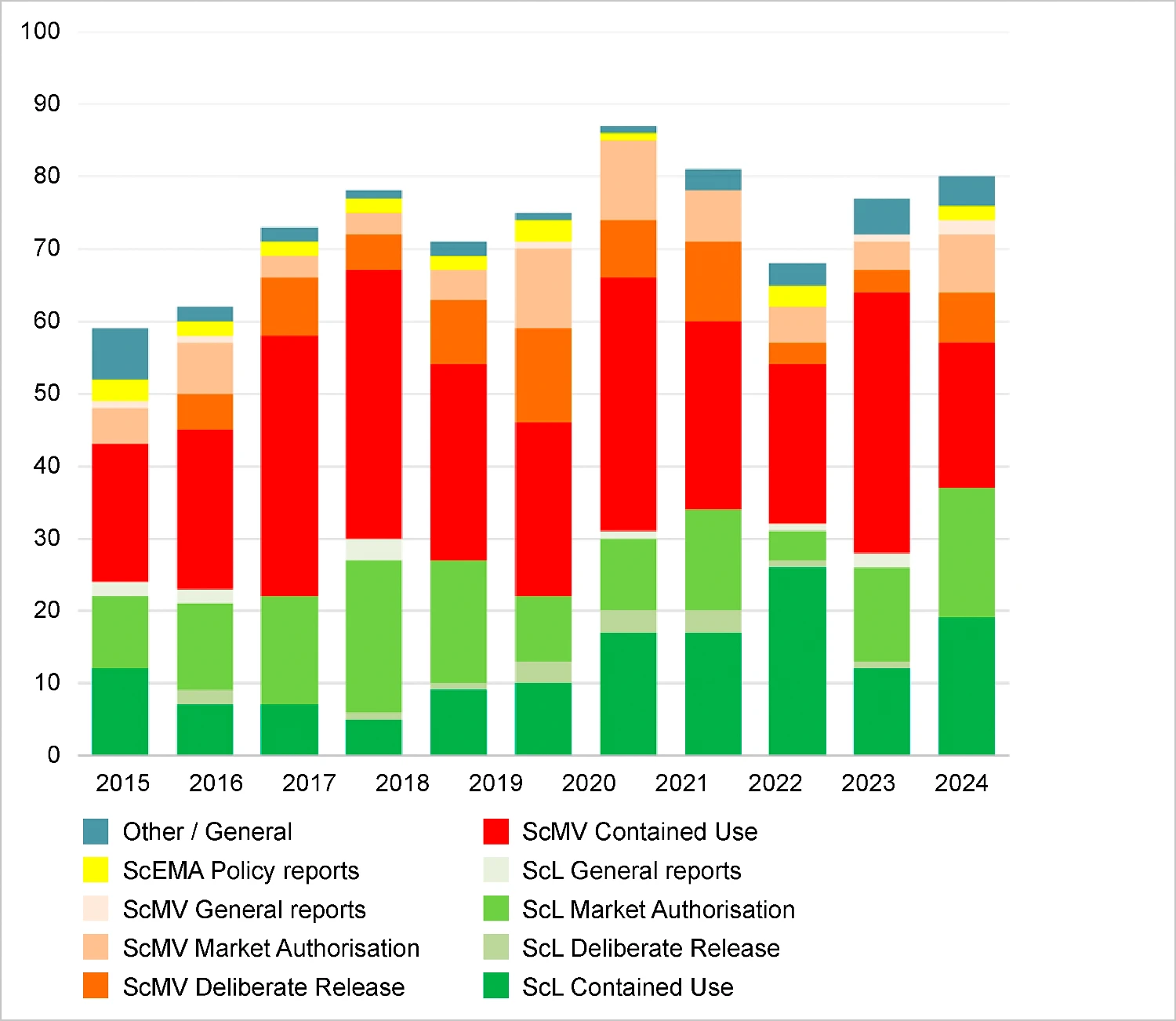
The number of advisory reports on applications for marketing authorisation was higher than in previous years. The number of reports on contained use activities was lower, due to a steep decline in the number of requests for advice within the remit of the Subcommittee on Medical and Veterinary Aspects concerning licence applications for experiments in laboratories and such like. The number of requests for advice on containment measures for genetically modified (GM) plants in greenhouses increased slightly. The rise in requests for advice on marketing authorisation increased across the board, although the biggest rise was for requests for advice on European marketing authorisation for GM crops. In addition, there was a slight increase in the number of requests for advice on gene therapy trials (figure 1).
One of the highlights last year was the completion of a policy report on the consequences for the healthcare budget of the growth in the number of gene therapies. The downside of the success of gene therapies is their high cost; the asking prices are the highest of all medications on the market. The policy report, which was prepared by the Subcommittee on Ethics and Societal Aspects (ScEMA), observes that the number of available gene therapies will rise rapidly over the coming years and that there is no sign of costs falling. When considering how to respond to this situation, the government should not focus solely on the very expensive drugs targeted at very small numbers of patients; it is precisely the less expensive treatments for large groups of patients, such as treatments for cancers, that have the greatest impact on the healthcare budget. This presents the government with painful choices and dilemmas if to wants to keep healthcare affordable.
Research reports
Three research reports commissioned by COGEM were published in 2024. These reports deal with the newest developments in GM bacteriophages, the possible risks of endogenous retroviruses in cell lines and possible changes in the presence of GM food products in Dutch supermarkets. All three reports are in English and can be downloaded from the COGEM website. A number of other research reports were completed in 2024, but have not yet been published. These reports will be published in the course of 2025.
Members
Three members stood down in 2024: Hans van den Berg MSc, Emeritus Professor Dick van Elsas and Emeritus Professor Arend Jan Waarlo. COGEM was pleased to welcome three new (associated) members in 2024: Hans Breeuwer PhD (University of Amsterdam), Marc van Mil PhD (UMC Utrecht) and Martina Sura-De Jong PhD (Van Hall Larenstein).
